Pvt Relationship For Polytropic Process
Compression theory is derived from the laws of thermodynamics.

Pvt relationship for polytropic process. Determine the final volume, in m3, and the work for the process, in kJ. From this relationship we can arrive at relationships for several other types of thermodynamic process:. PVT Relationships for Isentropic, IG Processes:.
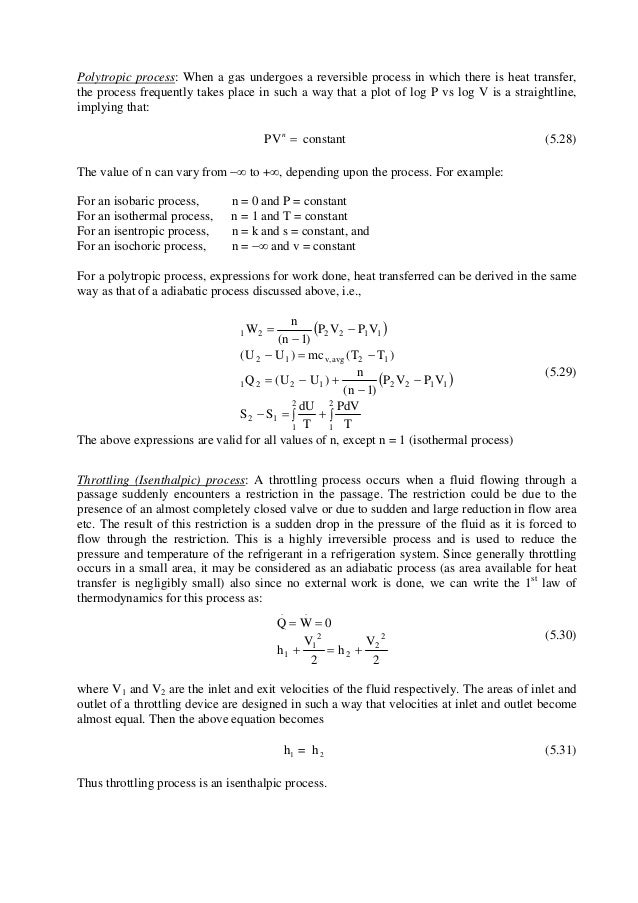
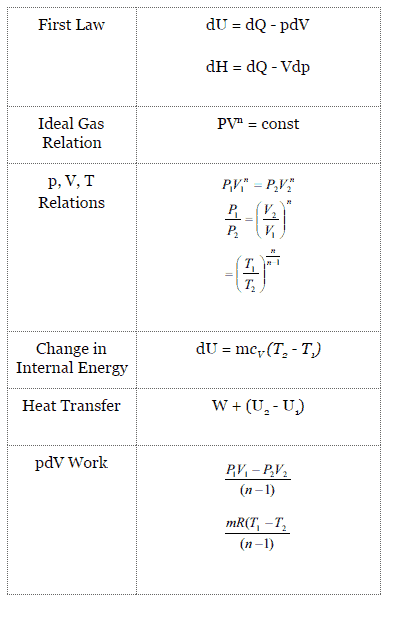
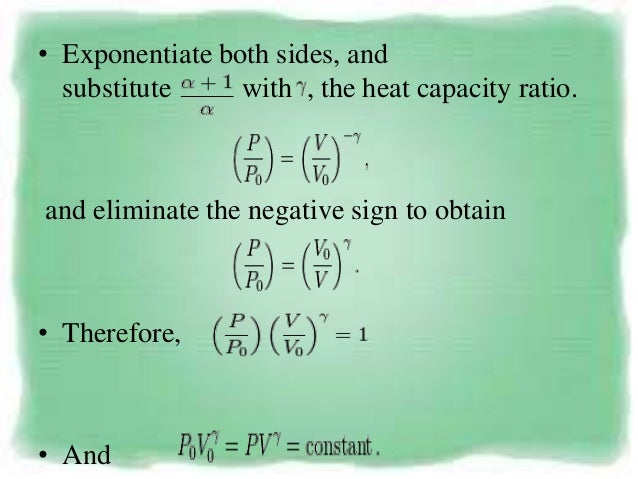
In each case the polytropic coefficient must be determined experimentally by measurement of the heat and work transfer and the initial and final states. The mathematical equation for an ideal gas undergoing a reversible (i.e., no entropy generation) adiabatic process can be represented by the polytropic process equation =, where P is pressure, V is volume, and for this case n = γ, where = = +, C P being the specific heat for constant pressure, C V being the specific heat for constant volume, γ is the adiabatic index, and f is the number of. = where p is the pressure, V is volume, n is the polytropic index, and C is a constant.
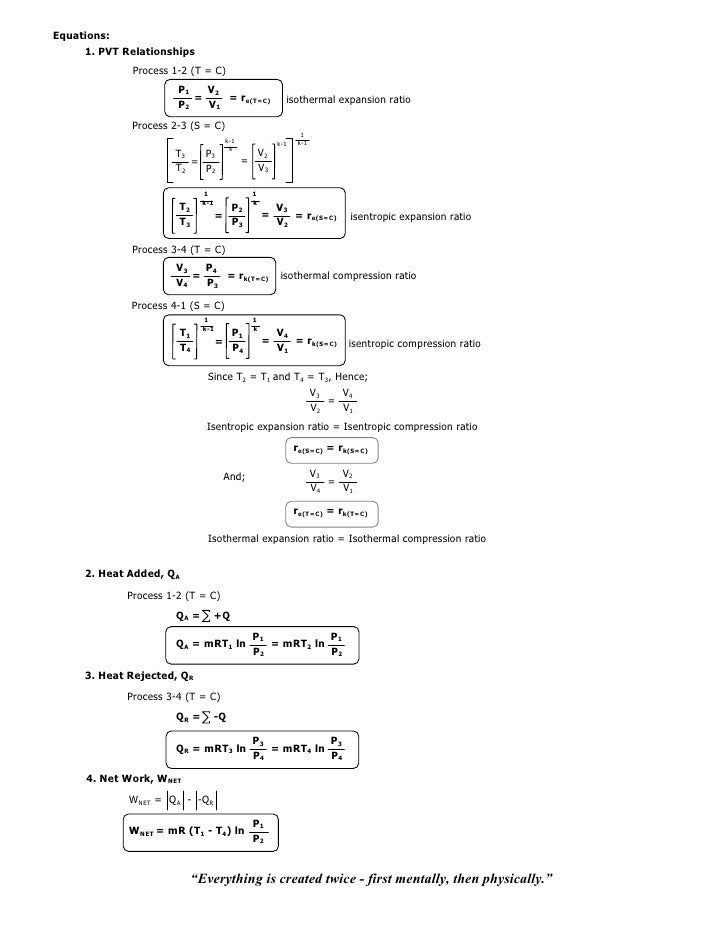
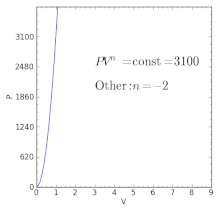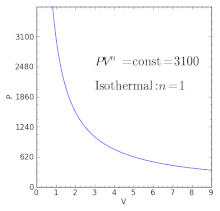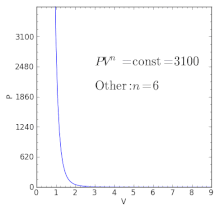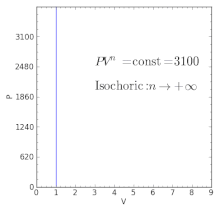
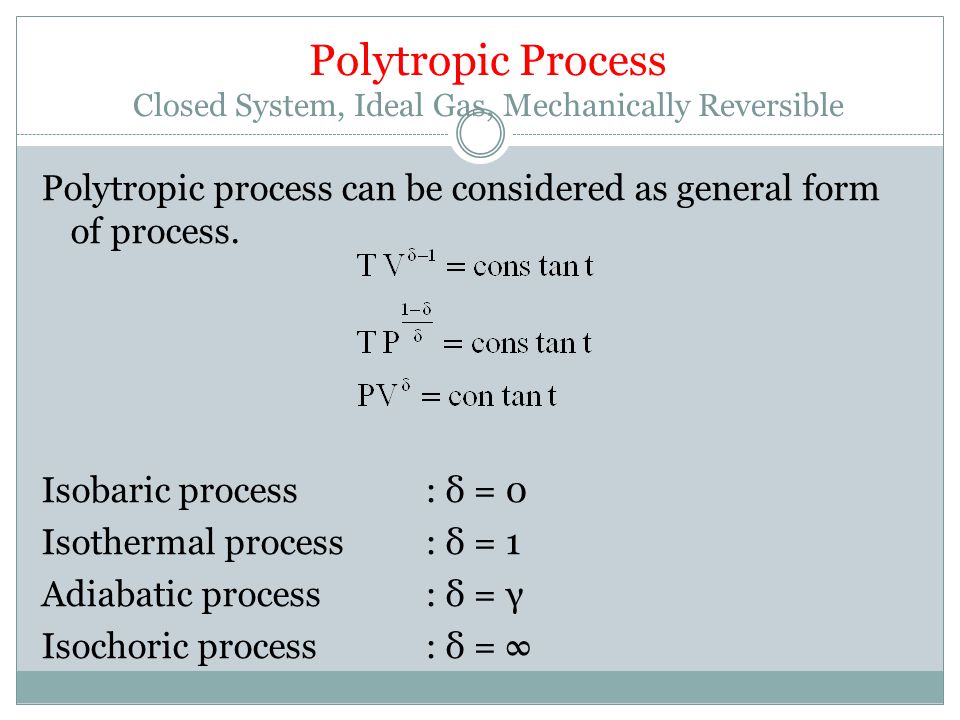
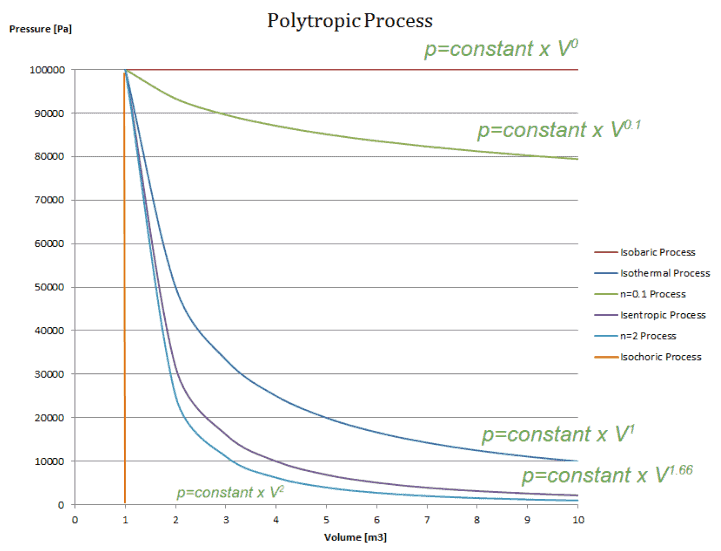
The condition of n = 0 represents an isobaric process, n = 1 represents an isothermal process, n = γ = cp / cv represents an isentropic process, and n = ∞ represents an isochoric process. Special Cases n =1 Pv= RT. Since the internal energy of an ideal gas depends on temperature only and the temperature is constant along Process 1-2, U 2 = U 1 and the energy balance reduces to:.
The air is now compressed slowly in a polytropic process according to the relationship Pvt = constant. When , the process is isobaric When , the process is isothermal When , the process is isentropic When , the process is isochoric Reversible. PV= RT(2) (n =1).
A gas in a piston–cylinder assembly undergoes a process for which the relationship between pressure and volume is pVn = constant. • A thermodynamic process described by the above equation is called a Polytropic process. The part we are going to study is “a system”, and the rest is “the surrounding”.
PVT V T p V T pV a a a a a a = = = 2. One form of this relationship is given by the equation pVn = constant • where n is a constant for the particular process. Get more help from Chegg.
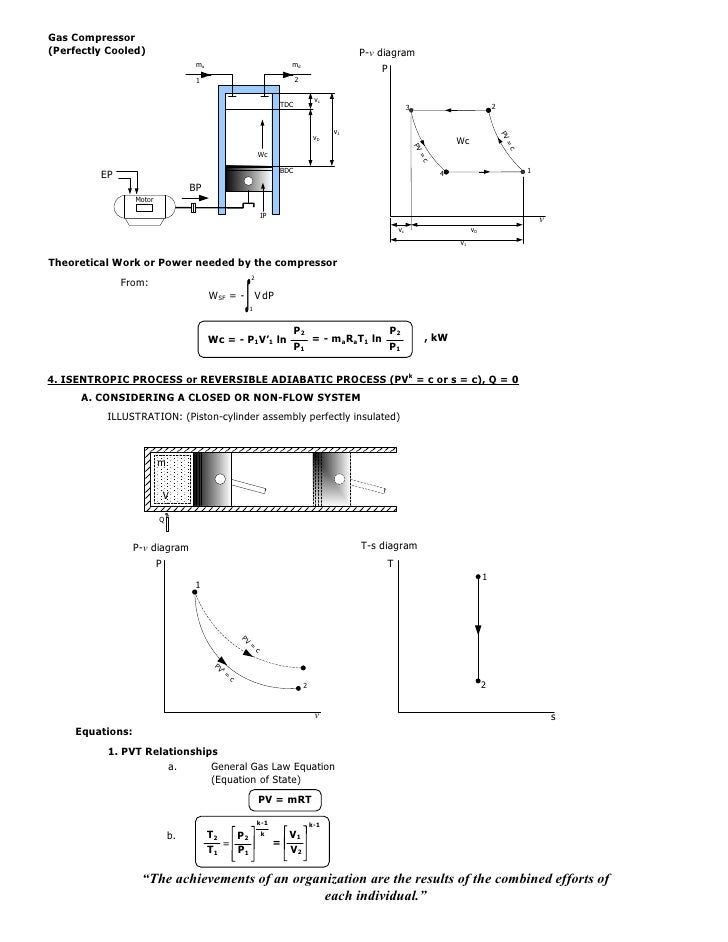
In chemistry, we divide the universe into two parts. P V k = constant where k is the coefficient of adiabatic compression or expansion. CYCLE FOR RECIPROCATING COMPRESSOR.
An adiabatic process is simply one that has no heat entering or leaving the gas, so the only energy going into or coming out of the gas is work. Definition and characteristics of Polytropic Process. Since in a quasi-static process a smooth graph can be drawn between pressure and volume therefore, can we conclude that all quasi-sta.
No heat transfer and no losses). The polytropic process equation can describe multiple expansion and compression processes which include heat transfer. The two processes used to calculate thermodynamic relationships are isentropic (adiabatic) and polytropic.
Pvk = Const (4.1.9) k is called the polytropic coefficient of the process. The basic "exponential definition" of path is defined analytically as a product of the ideal gas pressure times its volume with the volume itself enhanced by. Some of the more common values are given below.
V2 / V1 = ( p1 / p2 ) (1/n) T2 / T1 = ( p2 / p1 ) (n-1)/n. Relation between PVT Gas Laws:. That is to say, there is no.
The polytropic process is one in which the pressure-volume relation is given as. THERMODYNAMICS, HEAT TRANSFER, AND FLUID FLOW Rev. The polytropic index is 1.2 for the compression and expansion.
The outlet pressure is 8 bar. Assume d = 1.38 for this process path and that air behaves as an ideal gas.:. Many processes which occur in practice can be described approximately by an equation of the form PV n = constant, where n is a constant.
The Thermodynamics, Heat Transfer, and Fluid Flow Fundamentals Handbook was developed to assist nuclear facility operating contractors provide operators, maintenance. One of the great interests of the polytropic concept lies in the simplicity of equation. A polytropic process is any thermodynamic process that can be expressed by the following equation:.
A PVT relationship is one of the forms of the equations of state (see Equations of State), which relates the pressure, molar volume V and the temperature T of physically homogeneous media in thermodynamic equilibrium. The polytropic process is a modification of the adiabatic process, involving an efficiency to more closely represent actual conditions. The isentropic process is one in which the entropy of the system remains constant.
P V n = C. Polytropic Process During expansion and compression processes of real gases, pressure and volume are often related by PVn=C, where n and C are constants. General Gas Law Equation (Equation of State) PV = mRT k-1 k-1 b.
P-V-T Behavior of Pure Substances PT Diagram • A typical P-T diagram showing the relationship between pressure and temperature of a pure substance is shown below:. For this gas, C_ p = 29.1\ \frac{J}{mol.K}. Ideally the process 2 to 3 and 4 to 1 are isothermal.
The key difference between adiabatic and polytropic processes is that in adiabatic processes no heat transfer occurs whereas in polytropic processes heat transfer occurs. A polytropic process describes the transition of a fluid from one state to another through a specific relationship between the fluid density and temperature. A polytropic process is an approximation, much like a linear regression.
A polytropic process for a gas is one in which a gas is compressed or expanded, heated or cooled. Isentropic compression is also polytropic. You cannot use the relative pressure to solve the other parts of this problem.
There's the isothermal process where temperature is constant internal energy is constant, and the quantity P x V, pressure times volume, is also constant. {\displaystyle pV^ {\,n}=C} where p is the pressure, V is volume, n is the polytropic index, and C is a constant. The exponent n may have any value from minus infinity to plus infinity depending on the process.
The inlet conditions are 1 bar and 10oC. This condition can be used to derive the expression for the work done during an. The value of the polytropic index that governs this relationship determines the heat transfer and the effective degrees of freedom during the process.
Lesson E - Polytropic and Isentropic Processes. δQrev=0 We can now conclude from the above…. (a) Relation for Polytropic Processes:.
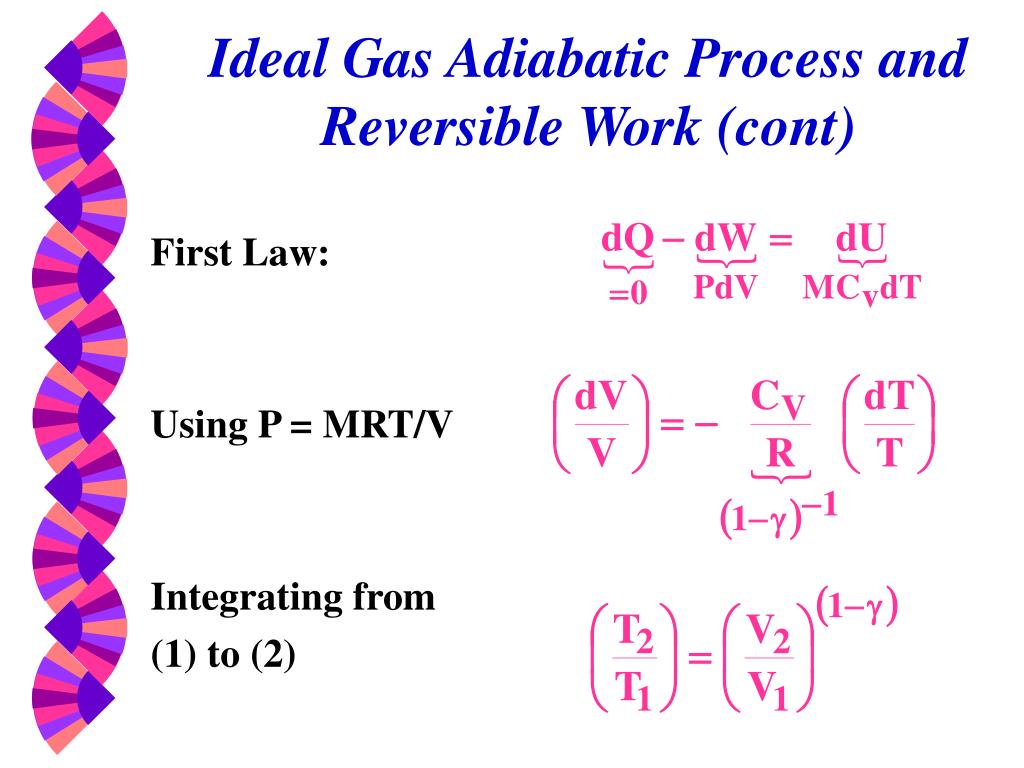
Polytropic Process of an Ideal Gas • The relationship between the pressure and volume during compression or expansion of an ideal gas can be described analytically. • the gas undergoes an isentropic process → reversible + adiabatic Combining this result with the ideal gas equation of state T 2 T 1 = v 1 v 2 k−1 = P 2 P 1 (k−1)/k The isentropic process is a special case of a more general process known as a polytropic process where → Pvn = constant and n is any number. 2 2 1 2 2 2 2 2 1 1 1 2 1 0.
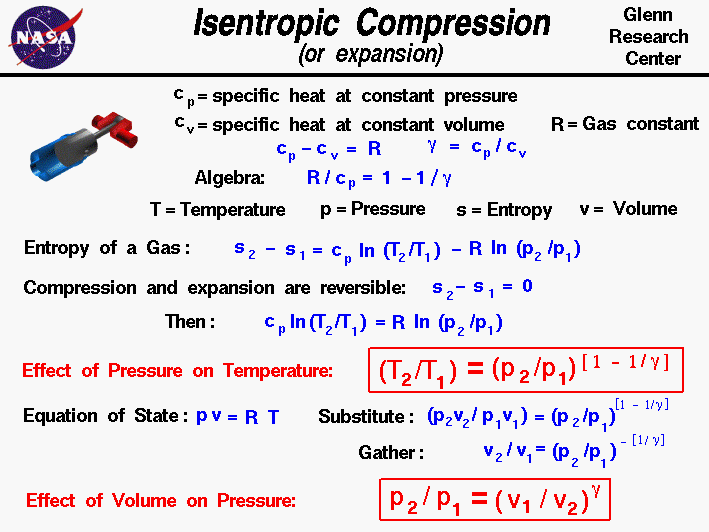
According to the definition of entropy which is expressed as:. Adiabatic relationship between P and V. For an isentropic process (dS=0), we obtain:.
PV Constant, where p v C C Applying first law of thermodynamics for an adiabatic process, we get:. Only system (d) is an open system with a fixed boundary because here mass flow and energy transfer both are taking place so option (d) will be answer. For an ideal gas, it can be shown that:.
Assume that the specific heats are constant. The final pressure is twice the original pressure. An isentropic process is adiabatic and reversible (i.e. The polytropic process is a widely used pressure model for predicting the nature of pressure and volume states in expansion and compression systems.
For a fluid undergoing an adiabatic process, the pressure and volume satisfy the following relation:. The moving work for a polytropic process can be found:. Three moles of an ideal gas are taken around the cycle abc shown in the figure.
3) All Systems (a) to (c) are closed systems, so we can’t use control mass for these cases. According to the first law of thermodynamics :. If the Process is Quasi-static (the intermediate Position is also in equilibrium).
A reversible process is one which is performed as if it were always at equilibrium, and without the production on entropy. A lot of expansion and compression processes follow this relationship. The area under a process curve on a T-s diagram is related to the amount of heat transferred to the gas.
Δs=s1-s2=0 This can also be described in the T-s diagram:. For the non-isentropic processes, I suggest you use a polytropic PVT relationship from page 186 in the TCD Notebook. For very small movement dx of Piston from this intermediate state F = PA → acting on Piston.
Each of the four processes which have been previously considered is a special case of a polytropic. (T2 / T1 ) (n/(n-1))= P2 / P1. DU = nCvdT, and Cv = dU/dT => dU = nCvdT = CvdT (as n=1) Also, for an adiabatic process, dQ = 0;.
The polytropic process equation can describe multiple expansion and compression processes which include heat transfer. The key to this problem is the fact that the process is polytropic and that the air can be assumed to be an ideal gas. Can we say all quasi-static processes are poly-tropic process?.
It we draw a graph between pressure and volume and consider a small part of this graph, the area of small element on volume axis is. The pressure-volume diagram of a Carnot power cycle executed by an ideal gas with constant specific heat ratio γ is shown in the diagram. In this study, we analyze solar wind.
RoyMech.co.uk has a decent article on it. It is correct to the extent by which the real and approximate curves are similar, however you define "similar". You may be missing something else.
Adiabatic process Polytropic process Constant Volume Process Throttling Process. (8) and (9) Many gas compression and expansion processes may be usefully approximated by a polytropic process. Where p is pressure, v is volume, n and C are constants.
The value of the polytropic exponent is n = 1.9. There's the isometric process, also known as isochoric or isovolumetric, where the change in volume is 0, which meant, remember, that means no work can be done. For ideal gases, sometimes with constant specific heats, you can derive certain polytropic relations,.
N PV PV W PdV CV ndV polytopic 1 2 2 1 1 2 1 1 Since PV n PVn C 1 1 2 2. Determine the final temperature (10), the work done (10) and heat transferred (15) for this process. This puts a constraint on the heat engine process leading to the adiabatic condition shown below.
PVT Relationships for Isentropic, IG Processes 8 pts;. DQ = dU + dW….(1) For one mole of gas, the equation is:. Calculate Q & W S, in kJ/kg, when ambient air at 104 kPa and 3 K is compressed polytropically to 950 kPa.
4.1.3.2.2 Integral approach of the polytropic The assumption that we make here is to apply the law followed by the fluid between the compressor inlet and outlet, which is of type:. \H_p = \int_1^2 vdP\ From the polytropic_relationships given in. An isentropic process appears as a vertical line on a T-s diagram.
N = polytropic exponent (or index) pVn= constant. Using the same relationship, the polytropic efficiency can be determined. The two variable nature of this model typically.
The stratagem "polytropic process," is an "exponent controlled," analytic assumption to define ideal processes of an ideal gas as having continuous (equilibrium path) of ideal gas states. Relationship between Pressure (P), Volume (V), Temperature (T) and quantity;. For axial-flow turbines, this means the engine system is neither gaining nor loosing energy, and whereby the sum total energies of all inputs equals that of the out.
Because for isolated system ,system should be a closed system at first. Isentropic Relations An isentropic process is a process during which the entropy of a system remains constant:. Stack Exchange network consists of 176 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers.
A polytropic process obeys the relation (6.157)pvn = C where v = 1 / ρ is the specific volume, n is the polytropic index, and C is a constant. T2 P2 k V1 = = T1 P1 V2 “The achievements of an organization are the results of the combined efforts of each individual.” 4. Process ac is at constant pressure, process ba is at constant volume, and.
Such a process is called a polytropic process. A polytropic process is a thermodynamic process that obeys the relation:. Because the process is polytropic, we can determine T 2 and W S.
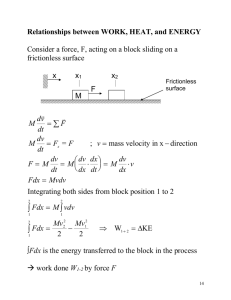
δ W = F×dx δ W = P Adx δ W = P dv. Adiabatic Processes If a material undergoes a change in its physical state (e.g., its pressure, volume, or temperature) without any heat be-. According to the ideal gas equation:.
You can use P1/P2=Pr (T1)/Pr (T2) for part (a) because the process is isentropic. The theory is applicable to both positive displacement and continuous flow compressors. The equivalent pressure—temperature and work relationships are as follows:.
PVT behaviour of gases and relations. A polytropic process is a thermodynamic process that obeys the relation:. Adiabatic Process An adiabatic process is one in which no heat is gained or lost by the system.
Now, having established a relationship between \(v\) and \(P\) for a polytropic process, the integral in the polytropic head expression can be evaluated. Moles (n) Boyle’s Law (PV = constant). Assumptions in Thermodynamic Cycles.
The first law of thermodynamics with Q=0 shows that all the change in internal energy is in the form of work done. It is possible to perform a series of processes, in which the state is changed during each process, but the gas eventually returns to its original state. The initial pressure is 1 bar, the initial volume is 0.12 m3, and the final pressure is 9 bar.

Thermodynamics By S K Mondal Copy
Neit Instructure Com Files Download Download Frd 1

Heatenginesvol 1 Chapter 2 Rs Heat Enthalpy
Pvt Relationship For Polytropic Process のギャラリー

Thermofluids Isometric Isobaric Polytropic And Adabaric Process
2

Charles Law An Overview Sciencedirect Topics

Solved Air Is Compressed From An Initial State Of 1 Atm A Chegg Com
Introduction
Nptel Ac In Content Storage2 Courses Pdf Rac lecture 5 Pdf
What Is Polytropic Process Quora
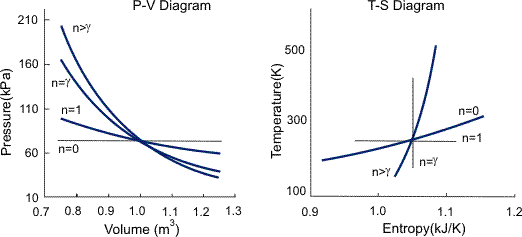
Polytropic Process
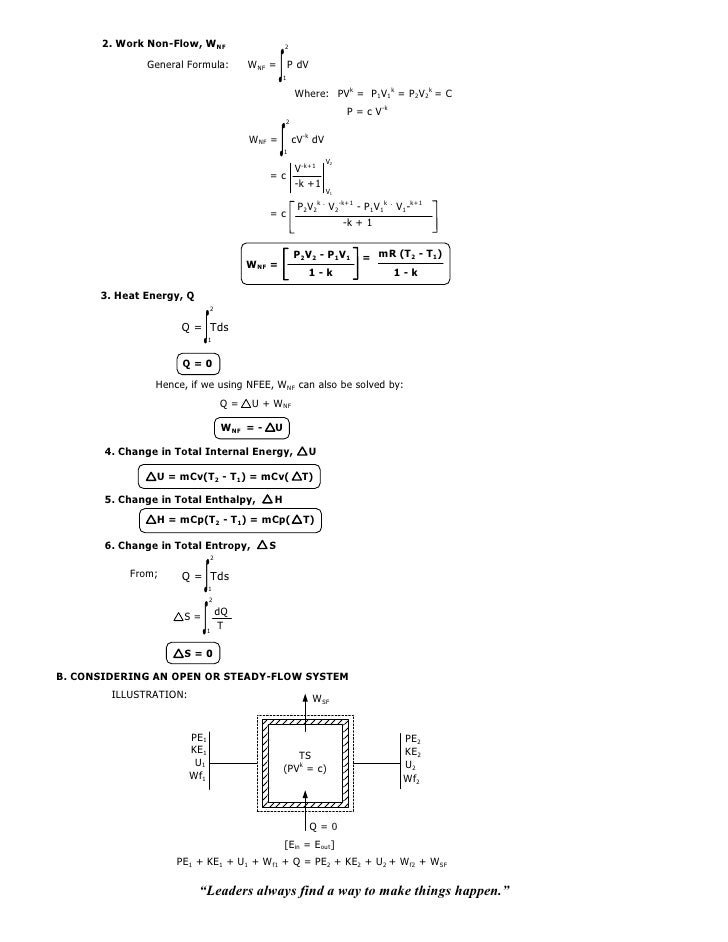
008 Isothermal Isentropic Polytropic Process
2
Nptel Ac In Content Storage2 Courses Pdf Rac lecture 5 Pdf

Thermodynamics
What Is Polytropic Process Quora
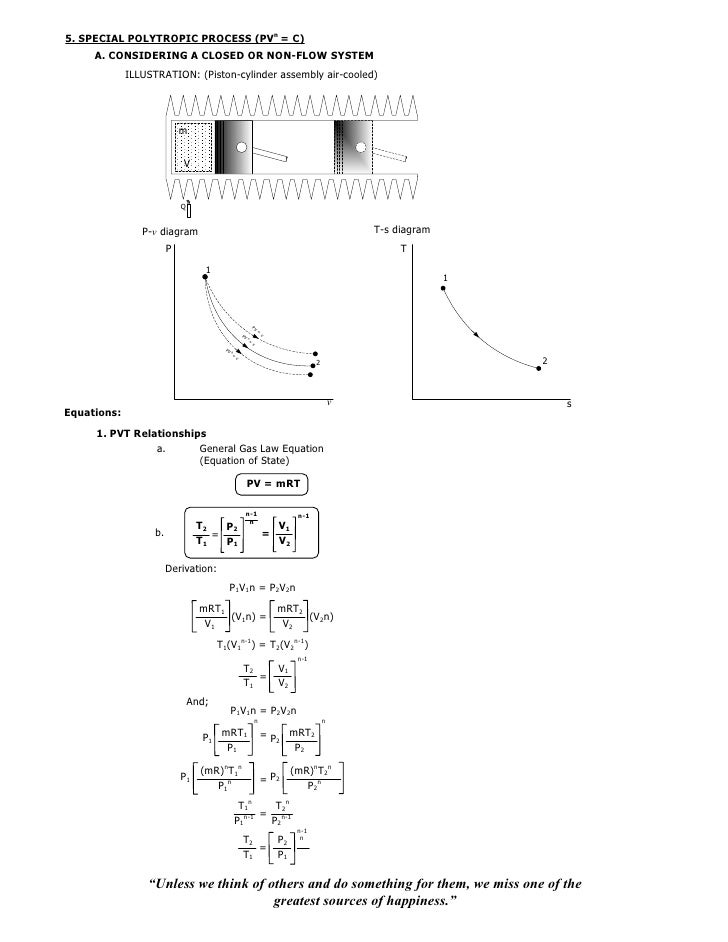
008 Isothermal Isentropic Polytropic Process
Bestgateiescoaching Files Wordpress Com 15 06 Chemical Engineering Thermodynamics Sample Chapter Pdf

Pdf Average Plasma Sheet Polytropic Index As Observed By Themis
2

Search Youtube Channels Noxinfluencer

Thermodynamic Relationship An Overview Sciencedirect Topics
Http Ska10 Weebly Com Uploads 4 7 1 1 Unit Iv Pdf

Adiabatic Process P V T Relation Youtube
Nanopdf Com Download Polytropic Process Of An Ideal Gas Pdf

Chapter7 Lesson E Pvt Relationships For Isentropic Ig Processes

008 Isothermal Isentropic Polytropic Process

Diesel Cycle Diesel Engine
Nptel Ac In Content Storage2 Courses Pdf Rac lecture 5 Pdf

Chapter8 Lesson B Polytropic Compression Of Air
What Is Polytropic Process Quora

Polytropic Process As A General Process Thermodynamics Youtube
Q Tbn 3aand9gcqhhahtu0h92a1p0 Lun7ijw9d6p8lxthsm30pyypg5xdws6i3e Usqp Cau
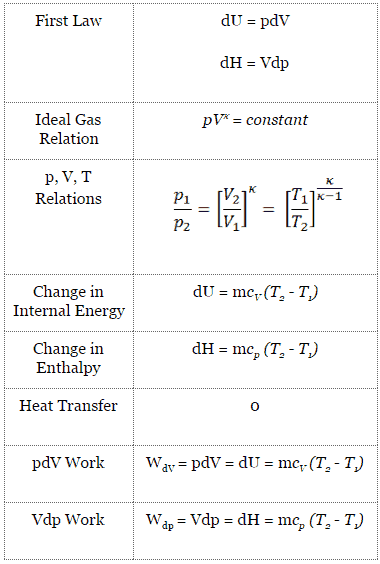
What Is Adiabatic Process Definition

Isentropic Process Nuclear Power

13 Thermodynamics Proof Of Adiabatic Equation Most Important Complete Concept Youtube

Thermodynamic Processes And Equations

Solved 2 5 Lbm Of Air Is In A Piston Cylinder Device Init Chegg Com

Rac Lecture 5

271f10l12 Physics Labs

What It The Temperature And Volume Change After Air Is Compressed From 1 Ksi To 10 Ksi Thermodynamics
008 Isothermal Isentropic Polytropic Process

011 Second Law Cycle Analysis

Change Of Entropy During Polytropic Process Youtube
1 Thermodynamics Thermodynamics Is The Branch Of Science

Adiabatic Process Relation Between P V And T Testbook
2

Equation Of State Wikipedia

Doc Processes Joven Estilo Embanecido Academia Edu
Rac Lecture 5
Q Tbn 3aand9gcr1uqvzwtyprcrsz0eegciwxc9dtnudebgknmhw60aqvhoivuq9 Usqp Cau

Experimental Theory Polytropic Process Docx Pressure Thermometer

Chapter 7 Entropy A Measure Of Disorder Study Guide In Powerpoint To Accompany Thermodynamics An Engineering Approach 6th Edition By Yunus A Cengel Ppt Download

Solved 2 5 Lbm Of Air Is In A Piston Cylinder Device Init Chegg Com
Polytropic Process Wikipedia

Polytropic Processes For An Ideal Gas Youtube
What Is Polytropic Process Definition

Why Is Pv Gamma Constant In An Adiabatic Process Physics Stack Exchange
Ppt Thermodynamic Properties Powerpoint Presentation Free Download Id 5036

The Polytropic Process

Thermodynamics Lecture Notes Ea1223 Studocu

Pvt Behaviour Of Gases And Relations

Rac Lecture 5

Thermodynamic Relationship An Overview Sciencedirect Topics
Q Tbn 3aand9gctpbf Q4twtzr Wzrn21ujwesazfz Ozh3wmqesohixux0ws60v Usqp Cau
Isentropic Compression Or Expansion

Thermodynamics Lecture 10 Polytropic Processes Youtube
Pvt Behaviour Of Gases And Relations
011 Second Law Cycle Analysis

Isobaric Process Wikipedia

Thermal Engineering 10
Http Www Mhtlab Uwaterloo Ca Courses Me354 Lectures Pdffiles Weball Pdf

Chapter 12 Engineering Thermodynamics
2

Ch Thermodynamics By Career Avenues Issuu

Thermodynamic Processes And Equations
2

Proof Of Pressure Volume And Temperature Ratio Adiabatic Process Youtube

The Polytropic Process

Shortcuts To Convert P V Diagram Into T S Diagram Exergic

The Residuals Plotted Against A Volume B Pressure And C Download Scientific Diagram

Ch7 Lesson E Page 13 Polytropic Paths Summary

Example 7e 4 Performance Of An Ideal Gas Cycle
2
Q Tbn 3aand9gcqxbypmefsvwvlwh Alivwm3bswl5fqpkrnkw Usqp Cau

The Polytropic Process Ideal Gas University Of Idaho Powerpoint Presentation Free Online Download Ppt Qp0ikk

First Law For Ideal Gases Thermodynamics Lecture Slides Docsity

The Relation Between The Temperature And Volume In Adiabatic Process Download Scientific Diagram

Chapter8 Lesson B Polytropic Compression Of Air

Polytropic Processes Definition Examples Diagrams
Q Tbn 3aand9gctwnso2 5yrzerdqkom2qclfec4ne2bszff6bzuwwh Kgeb3v01 Usqp Cau

Equation Of State Wikipedia
Process Calculation In Ideal Gas

Lecture Notes 3 Gas Compressor Refrigeration
Itk 233 Termodinamika Teknik Kimia I Ppt Video Online Download

What It The Temperature And Volume Change After Air Is Compressed From 1 Ksi To 10 Ksi Thermodynamics

Adiabatic Process Relation Between P V And T Testbook

Adiabatic Process P V T Relation Youtube

Thermodynamic Properties Property Relationships And Processes

Ch7 Lesson E Page 19 Heat Work In Int Rev Polytropic Processes
What Is Polytropic Process Definition

Experimental Theory Polytropic Process Docx Pressure Thermometer

Thermo Objective Pages 1 50 Text Version Anyflip

What Is The Relation Between Temperature Pressure And Volume In An Adiabatic Process Thermodynami Youtube




